How Long Does It Take to Read a Tb Test
| Mantoux test | |
|---|---|
 The Mantoux peel test consists of an intradermal injection of one-tenth of a milliliter (ml) of PPD tuberculin. The circular shape is known every bit a wheal response. | |
| Synonyms | Mantoux screening test |
| Purpose | screen for tuberculosis |
The Mantoux test or Mendel–Mantoux test (also known as the Mantoux screening test, tuberculin sensitivity test, Pirquet exam, or PPD test for purified protein derivative) is a tool for screening for tuberculosis (TB) and for tuberculosis diagnosis. Information technology is i of the major tuberculin skin tests used effectually the world, largely replacing multiple-puncture tests such as the tine exam. The Heaf test, a form of tine examination, was used until 2005 in the Great britain, when it was replaced by the Mantoux exam. The Mantoux exam is endorsed by the American Thoracic Society and Centers for Disease Control and Prevention. It was also used in the USSR and is at present prevalent in most of the post-Soviet states.
History [edit]
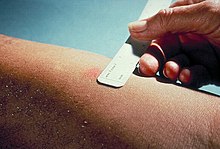
The size of induration is measured 48–72 hours subsequently. Erythema (redness) should not exist measured.

Mantoux examination injection site in a subject without chronic atmospheric condition or in a high-risk group clinically diagnosed as negative at 50 hours
Tuberculin is a glycerol extract of the tubercle bacillus. Purified protein derivative (PPD) tuberculin is a precipitate of species-nonspecific molecules obtained from filtrates of sterilized, concentrated cultures. The tuberculin reaction was beginning described by Robert Koch in 1890. The test was first adult and described by the German physician Felix Mendel in 1908.[i] It is named after Charles Mantoux, a French physician who built on the work of Koch and Clemens von Pirquet to create his examination in 1907. However, the examination was unreliable due to impurities in tuberculin which tended to cause false results.[2]
Esmond R. Long and Florence B. Seibert identified the active amanuensis in tuberculin equally a protein. Seibert then spent a number of years developing methods for separating and purifying the poly peptide from Mycobacterium tuberculosis, obtaining purified poly peptide derivative (PPD) and enabling the cosmos of a reliable test for tuberculosis.[two] Her kickoff publication on the purification of tuberculin appeared in 1934.[3] By the 1940s, Seibert'due south PPD was the international standard for tuberculin tests.[4] In 1939, Thou. A. Linnikova in the USSR created a modified version of PPD. In 1954, the Soviet Union started mass production of PPD-50, named after Linnikova.[5] [6]
Procedure [edit]
In the Mantoux test, a standard dose of five tuberculin units (TU - 0.i ml), co-ordinate to the CDC,[vii] or two TU of Statens Serum Institute (SSI) tuberculin RT23 in 0.1 ml solution, co-ordinate to the National Health Service,[8] is injected intradermally (between the layers of dermis) on the flexor surface of the left forearm, mid-way between elbow and wrist. The injection should be made with a tuberculin syringe, with the needle bevel facing upward. Alternatively, the probe can be administered by a needle-costless jet injector. When placed correctly, injection should produce a pale wheal of the peel, 6 to ten mm in bore. The result of the examination is read after 48–96 hours but 72 hours (3rd day) is the ideal. This intradermal injection is termed the Mantoux technique. A person who has been exposed to the bacteria is expected to mount an immune response in the skin containing the bacterial proteins. The response is a classical instance of delayed-blazon hypersensitivity reaction (DTH), a type 4 of hypersensitivities. T cells and myeloid cells are attracted to the site of reaction in the timeframe of 1–3 days and generate local inflammation. The reaction is read by measuring the diameter of induration (palpable raised, hardened area) across the forearm (perpendicular to the long centrality) in millimeters. If there is no induration, the upshot should be recorded as "0 mm". Erythema (redness) should not exist measured.[ citation needed ] In the Pirquet version of the test tuberculin is applied to the skin via scarification.[nine]
Nomenclature of tuberculin reaction [edit]
The results of this test must exist interpreted carefully. The person's medical risk factors determine at which increment (5 mm, 10 mm, or fifteen mm) of induration the result is considered positive.[10] A positive result indicates TB exposure.
- five mm or more than is positive in
- An HIV-positive person
- Persons with recent contacts with a TB patient
- Persons with nodular or fibrotic changes on chest Ten-ray consistent with old healed TB
- Patients with organ transplants, and other immunosuppressed patients
- x mm or more is positive in
- Recent arrivals (less than five years) from high-prevalence countries
- Injection drug users
- Residents and employees of high-risk congregate settings (e.g., prisons, nursing homes, hospitals, homeless shelters, etc.)
- Mycobacteriology lab personnel
- Persons with clinical conditions that place them at loftier risk (e.g., diabetes, prolonged corticosteroid therapy, leukemia, terminate-stage renal affliction, chronic malabsorption syndromes, depression body weight, etc.)
- Children less than four years of age, or children and adolescents exposed to adults in high-hazard categories
- fifteen mm or more is positive in
- Persons with no known risk factors for TB[11]
A tuberculin exam conversion is defined equally an increase of 10 mm or more than within a two-yr menstruation, regardless of age. Alternative criteria include increases of vi, 12, fifteen or 18 mm.[12]
Simulated positive result [edit]
TST (tuberculin peel test) positive is measured past size of induration. The size of the induration considered to be a positive result depends on adventure factors. For instance, a low-risk patient must accept a larger induration for a positive upshot than a high-chance patient. Loftier-risk groups include recent contacts, those with HIV, those with chest radiograph with fibrotic changes, organ transplant recipients, and those with immunosuppression.
According to the Ohio Department of Health and US Department of Health, the Bacillus Calmette–Guérin (BCG) vaccine does not protect against TB infection. Information technology does, though, give eighty% of children protection against tuberculous meningitis and miliary tuberculosis. Therefore, a positive TST/PPD in a person who has received BCG vaccine is interpreted as latent TB infection (LTBI).[13] Due to the test'due south low specificity, most positive reactions in low-risk individuals are false positives.[14] A false positive effect may be caused by nontuberculous mycobacteria or previous administration of BCG vaccine. Vaccination with BCG may result in a false-positive result for many years after vaccination.[15]
False positives can also occur when the injected area is touched, causing swelling and itching. If the swelling is less than five mm, it is possibly due to error by the healthcare personnel causing inflammation to the area.
Another source of simulated positive results can be allergic reaction or hypersensitivity. Although rare, (about 0.08 reported reactions per meg doses of tuberculin), these reactions tin be dangerous and precautions should be taken by having epinephrin available.[sixteen]
False negative result [edit]
Reaction to the PPD or tuberculin test is suppressed by the following conditions:
- Recent TB infection (less than eight–10 weeks)
- Infectious mononucleosis
- Live virus vaccine - The examination should non be carried out within 3 weeks of alive virus vaccination (e. 1000. MMR vaccine or Sabin vaccine).
- Sarcoidosis
- Hodgkin's disease
- Corticosteroid therapy/steroid employ
- Malnutrition
- Immunological compromise - Those on immuno-suppressive treatment or those with HIV and low CD4 T jail cell counts, oft evidence negative results from the PPD test.[ citation needed ]
This is because the immune system needs to be functional to mount a response to the protein derivative injected under the skin. A simulated negative result may occur in a person who has been recently infected with TB, but whose immune organisation hasn't still reacted to the leaner.
- Upper respiratory virus infection
In case a 2nd tuberculin test is necessary information technology should be carried out in the other arm to avoid hypersensitising the skin.
BCG vaccine and the Mantoux test [edit]
The role of Mantoux testing in people who have been vaccinated is disputed. The US recommends that tuberculin skin testing is not contraindicated for BCG-vaccinated persons, and prior BCG vaccination should not influence the interpretation of the test. The UK recommends that interferon-γ testing should be used to help interpret positive Mantoux tests of over 5mm[17], and repeated tuberculin pare testing must not exist done in people who have had BCG vaccinations. In general, the US recommendation may result in a larger number of people being falsely diagnosed with latent tuberculosis, while the UK approach has an increased gamble of missing patients with latent tuberculosis who should be treated.[ citation needed ]
Co-ordinate to the Us guidelines, latent tuberculosis infection diagnosis and treatment is considered for whatsoever BCG-vaccinated person whose skin test is 10 mm or greater, if whatever of these circumstances are nowadays:
- Was in contact with another person with infectious TB
- Was born or has lived in a loftier TB prevalence country
- Is continually exposed to populations where TB prevalence is loftier
Anergy testing [edit]
In cases of anergy, a lack of reaction by the body'due south defence mechanisms when information technology comes into contact with strange substances, the tuberculin reaction will occur weakly, thus compromising the value of Mantoux testing. For example, anergy is present in AIDS, a illness which strongly depresses the immune arrangement. Therefore, anergy testing is advised in cases where at that place is suspicion that anergy is present. However, routine anergy pare testing is not recommended.[18]
Two-step testing [edit]
Some people who have been infected with TB may accept a negative reaction when tested years after infection, as the immune organisation response may gradually wane. This initial skin examination, though negative, may stimulate (heave) the body's ability to react to tuberculin in time to come tests. Thus, a positive reaction to a subsequent test may exist misinterpreted every bit a new infection, when in fact information technology is the outcome of the additional reaction to an old infection.[19]
Utilize of two-step testing is recommended for initial peel testing of adults who will be retested periodically (e.g., health care workers). This ensures any future positive tests can exist interpreted every bit beingness caused past a new infection, rather than only a reaction to an quondam infection.
- The outset test is read 48–72 hours after injection.
- If the first examination is positive, consider the person infected.
- If the first examination is negative, requite a 2nd test one to three weeks after the outset injection.
- The 2nd test is read 48–72 hours after injection.
- If the second test is positive, consider the person infected in the distant by [20]
- If the 2d test is negative, consider the person uninfected.[21]
A person who is diagnosed as "infected in the distant past" on two-pace testing is called a "tuberculin reactor".
The Us recommendation that prior BCG vaccination be ignored results in nearly universal false diagnosis of tuberculosis infection in people who have had BCG (mostly foreign nationals).
The latest estimation for Mantoux test results [edit]
According to the guidelines published by Centers for Disease Control and Prevention in 2005, the results are re-categorized into 3 parts based on their previous or baseline outcomes:
- Baseline test: ≥10 mm is positive (either first or second pace); 0 to nine mm is negative
- Serial testing without known exposure: Increase of ≥10 mm is positive
- Known exposure:
- ≥v mm is positive in patients with baseline of 0 mm
- ≥10 mm is positive in patients with negative baseline or previous screening result of >0 mm
Contempo developments [edit]
In addition to tuberculin peel tests such as (principally) the Mantoux test, interferon gamma release assays (IGRAs) accept go common in clinical use in the 2010s. In some contexts they are used instead of TSTs, whereas in other contexts TSTs and IGRAs both go along to be useful.[22]
The QuantiFERON-TB Gold blood test measures the patient's allowed reactivity to the TB bacterium, and is useful for initial and series testing of persons with an increased risk of latent or active tuberculosis infection. Guidelines for its utilise were released by the CDC in Dec 2005.[23] QuantiFERON-TB Gold is FDA-approved in the United States, has CE Mark approval in Europe and has been approved past the MHLW in Japan. The interferon gamma release assay is the preferred method for patients who have had immunosuppression and are about to commencement biological therapies.[24]
T-SPOT.TB is another IGRA; it uses the ELISPOT method.
Heaf examination [edit]
The Heaf tuberculin skin test was used in the United kingdom, only discontinued in 2005. The equivalent Mantoux test positive levels done with 10 TU (0.ane ml at 100 TU/ml, i:1000) are[ citation needed ]
- <5 mm induration (Heaf 0–1)
- five–fifteen mm induration (Heaf 2)
- >fifteen mm induration (Heaf three–4)
Come across likewise [edit]
- Tuberculosis
- Latent tuberculosis
- QuantiFERON
- Tine test
References [edit]
- ^ F. Mendel. Therapeutische Monatshefte, Berlin, 1903, 16: 177. Die von Pirquet'sche Hautreaktion und dice intravenöse Tuberkulinbehandlung.Medizinische Klinik, München, 1908, 4: 402-404.
- ^ a b "Esmond R. Long and Florence B. Seibert". Chemical Heritage Foundation. Archived from the original on January xiii, 2012. Retrieved April 27, 2011.
- ^ "Florence Seibert, American Biochemist, 1897–1991". Chemistry Explained . Retrieved 26 October 2015.
- ^ Dacso, C. C. (1990). "Chapter 47: Pare Testing for Tuberculosis". In Walker, H. K.; Hall, West. D.; Hurst, J.W. (eds.). Clinical Methods: The History, Physical, and Laboratory Examinations (3rd ed.). Boston: Butterworths. ISBN9780409900774 . Retrieved 26 October 2015.
- ^ "Mantoux test,Mantoux test inventors". Edubilla.com . Retrieved 2019-04-25 .
- ^ "Mantoux test | Clinical Medicine | Medical Specialties". Scribd . Retrieved 2019-04-25 .
- ^ "TB Elimination - Tuberculin Skin Testing" (PDF). CDC.gov. CDC - National Eye for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention - Division of Tuberculosis Elimination. October 2011. Retrieved 5 June 2017.
- ^ "The Mantoux test: Assistants, reading and interpretation" (PDF). NHS.uk. Archived from the original (PDF) on 15 Feb 2010. Retrieved 5 June 2017.
- ^ "Pirquet's skin examination | medicine".
- ^ From the CDC squad of the CDC team at the Saskatchewan Lung Association, photos of a PPD bump Archived 2007-03-21 at the Wayback Motorcar.
- ^ Mantoux Test Archived 2016-09-30 at the Wayback Machine in eac.int.
- ^ Menzies, Dick (1 January 1999). "Interpretation aof Repeated Tuberculin Tests". American Journal of Respiratory and Critical Care Medicine. 159 (ane): xv–21. doi:10.1164/ajrccm.159.1.9801120. PMID 9872812.
- ^ Information as well from ODH lecture at the Ohio Country University 5/24/2012.
- ^ Starke JR (Jul 1996). "Tuberculosis Skin Testing: New Schools of Thought". Pediatrics. 98 (1): 123–125. doi:x.1542/peds.98.i.123. ISSN 0031-4005. PMID 8668383. S2CID 19907614.
- ^ Chaturvedi Due north, Cockcroft A (1992). "Tuberculosis screening among health service employees: who needs chest X-rays?". J Soc Occup Med. 42 (4): 179–82. doi:10.1093/occmed/42.4.179. PMID 1421331.
- ^ James E. Froeschle; Frederick L. Ruben; A. Michael Bloh (2002). "Immediate Hypersensitivity Reactions after Utilize of Tuberculin Skin Testing". Clinical Infectious Diseases. 34 (1): e12–e13. doi:10.1086/324587. PMID 11731966.
- ^ https://www.nice.org.uk/guidance/ng33/chapter/Recommendations#latent-tb
- ^ Markowitz, Norman (1993). "Tuberculin and Anergy Testing in HIV-Seropositive and HIV-Seronegative Persons". Ann Intern Med. 119 (3): 185–193. doi:10.7326/0003-4819-119-three-199308010-00002. PMID 8100692. S2CID 37590470.
- ^ "Fact Sheets | Testing & Diagnosis | Fact Canvas - Tuberculin Pare Testing | TB | CDC". www.cdc.gov. 2018-12-11. Retrieved 2019-05-29 .
- ^ "Information on Two-Step TB Skin Test" (PDF). Archived from the original (PDF) on 2020-08-03. Retrieved 2017-03-13 .
- ^ Part of Health and Human Services. "Booster Phenomenon". Retrieved 2008-07-02 .
- ^ Collins, LF; Geadas, C; Ellner, JJ (2016), "Diagnosis of latent tuberculosis infection: too soon to pull the plug on the tuberculin skin examination", Ann Intern Med, 164 (two): 122–124, doi:10.7326/M15-1522, PMID 26642354, S2CID 1059756.
- ^ Guidelines for Using the QuantiFERON-TB Aureate Exam for Detecting Mycobacterium tuberculosis Infection, U.s.
- ^ British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017* world wide web.bad.org.uk, accessed 11 Oct 2020
Source: https://en.wikipedia.org/wiki/Mantoux_test
0 Response to "How Long Does It Take to Read a Tb Test"
Post a Comment